What is male menopausal syndrome?

Treatment candidates
- Psychological symptoms such as insomnia and irritability
- Physical symptoms such as fatigue, headaches, and tinnitus
- Decline in concentration and memory
- Decreased sexual function, erectile dysfunction (ED)
Those ineligible for treatment
- Individuals under 18 years of age at the time of treatment application
- Individuals who are not in a health condition sufficient to withstand fat extraction
- Individuals who do not have the capacity to provide informed consent, or consent cannot be obtained from a surrogate decision-maker
- Individuals who have not accepted the informed consent document for this treatment, received sufficient explanation, and demonstrated consent by written means (surrogate decision-maker has not consented in writing)
- Individuals for whom the attending physician has determined that the treatment is not indicated based on medical history, examination, etc.
- Pregnant individuals
- Individuals undergoing treatment for gynecological disorders
- Individuals diagnosed with proliferative diabetic retinopathy or age-related macular degeneration
- Individuals with poorly controlled hypertension or arrhythmia
- Individuals exhibiting clinical symptoms of delirium
- Individuals with a hypersensitivity reaction to penicillin
- Individuals currently undergoing treatment for or hospitalized for cerebrovascular disorders such as stroke or intracranial hemorrhage within the past 3 months
- Individuals currently undergoing treatment for brain tumors, untreated depression, or depression that has not improved with treatment
- Individuals with positive results from infectious disease tests such as hepatitis B, hepatitis C, HIV, syphilis, etc. conducted within the past 12 weeks
※For more details, please inquire via phone or email.
Benefits of Adipose Stem Cell Therapy
1.Using one’s own adipose-derived stem cells ensures minimal side effects and safety.
Since no drugs or chemicals are used, you can undergo treatment with peace of mind.

2.No hospitalization or surgery is necessary.
Fat stem cell therapy can be performed on an outpatient basis, both for the collection of adipose tissue and the administration of autologous adipose-derived stem cells, resulting in minimal physical burden.
3.Storing stem cells enables lifelong treatment.
培Cultivated stem cells can be preserved for future use, allowing for the possibility of reinjection.
Treatment Process
Treatment is conducted on an outpatient basis, with no need for hospitalization.
STEP01
Consultation
After receiving thorough explanations, you will be asked to submit a consent form based on the treatment plan.

↓
STEP02
Blood Test
Preparatory tests including infection screenings via blood draw will be conducted. Results typically take about a week to be available.
↓
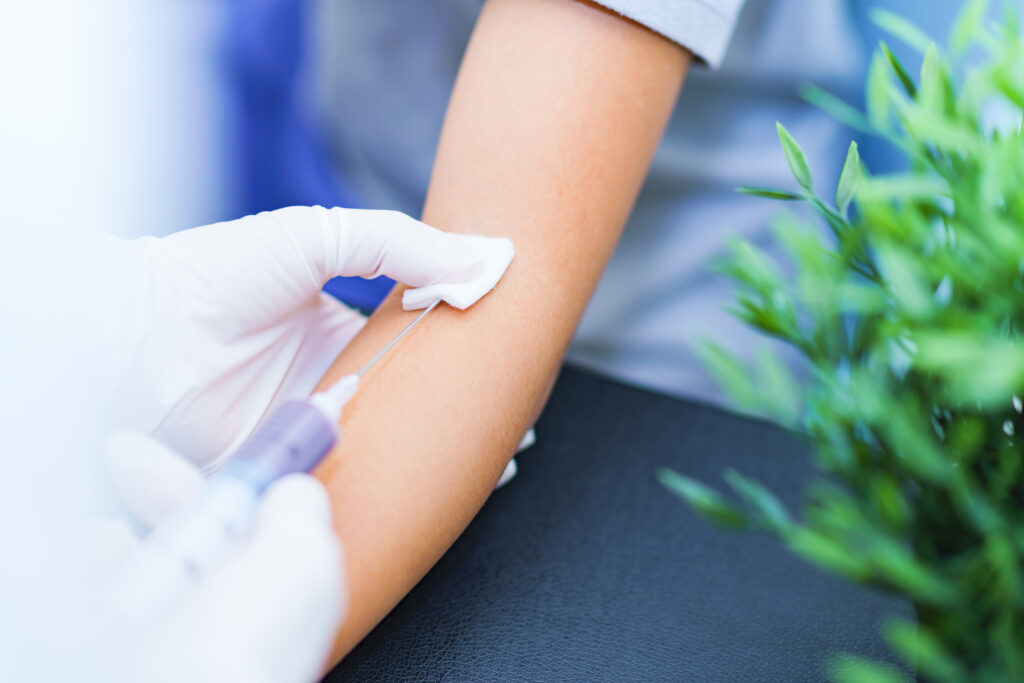
STEP03
Fat Extraction and Blood Collection
A small amount of fat will be collected from a location on the abdomen or similar area where fat can be reliably extracted under local anesthesia. Additionally, 100ml of blood will be drawn for cultivation purposes.
↓
STEP04
Cell Cultivation and Proliferation at CPC (Cultivation Facility)
※Approximately 6-8 weeks

↓
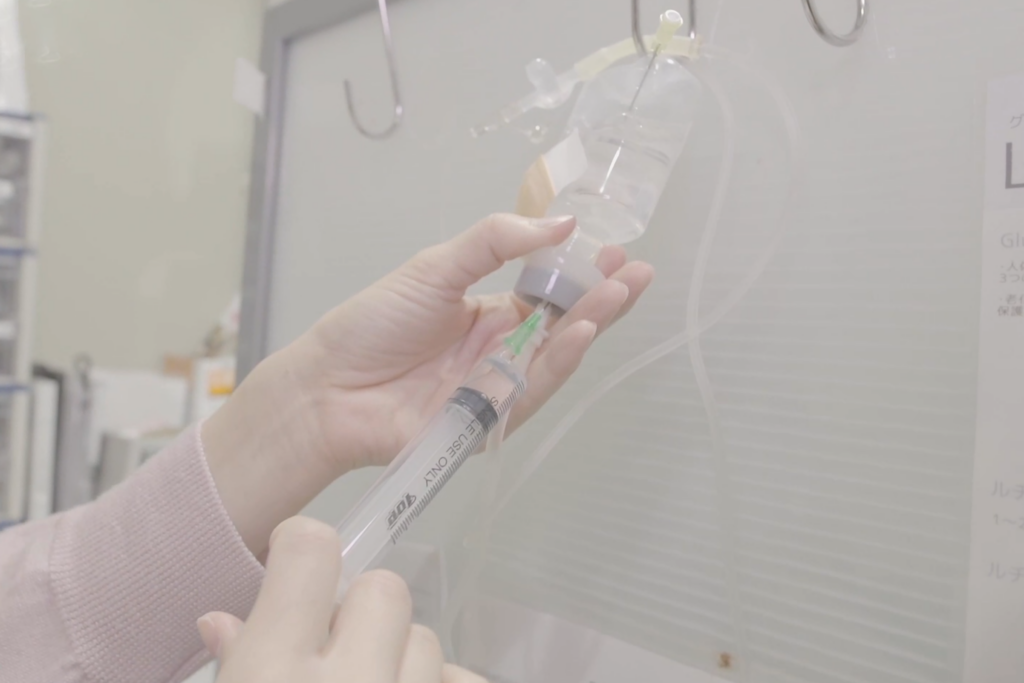
STEP05
Intravenous Infusion (Approximately 30 minutes)
The cultivated stem cells will be administered via intravenous drip.
↓
STEP06
Follow-up Observation
We will aim to observe the patient at 1, 3, 6, and 12 months after stem cell administration to the best of our ability.
Expected Effects and Potential Side Effects
Expected Effects
Adipose-derived mesenchymal stem cells are capable of differentiating into nerve, adipose, muscle, bone, cartilage, and other visceral tissues, acquiring their morphology and functions. They are believed to be capable of repairing damaged or aging cells. Additionally, secretions from stem cells can diffuse into the surrounding area and directly affect neighboring cells (hom ing effect), leading to various therapeutic effects such as immune regulation, angiogenesis, anti-inflammatory effects, antioxidant effects, anti-apoptotic effects, and tissue repair effects.
Potential Side Effects
【During Fat Tissue Extraction】
- Bleeding from the incision site
- Infection or pain at the incision site
- Side effects associated with anesthesia use, allergic reactions (such as palpitations, hives, difficulty breathing)
【Adverse Reactions During Administration】
- Fever after stem cell administration (typically resolves within 24 hours)
- Allergic symptoms: Reports of allergic symptoms such as palpitations, hives, and difficulty breathing during treatment.
- Unexpected adverse events: There have been cases where patients who received adipose-derived mesenchymal stem cell therapy died from pulmonary embolism. While the causal relationship between stem cell administration and death from pulmonary embolism remains unclear, pulmonary embolism is the most dangerous complication of intravenous stem cell administration.
If pulmonary embolism occurs during the course of treatment, we will respond as follows in our hospital
①If it occurs within the hospital, we will assess the severity based on the treatment guidelines for embryonic thromboembolism, promptly implement respiratory and circulatory management, and arrange emergency transport to affiliated medical institutions or others.
②If you experience sudden difficulty breathing, chest pain, or cold sweats outside of our clinic hours, please contact our clinic by phone (emergency contact outside of office hours). We will assess whether emergency care is necessary and provide guidance on referral to affiliated medical facilities if needed.
③For any adverse events, please contact our clinic first, and we will address them accordingly. If there are any symptoms of concern or discomfort following stem cell administration, please contact us. In the event that our clinic is unable to address the symptoms, we will provide guidance on referral to external medical facilities with fully equipped inpatient facilities.
Precautions for this treatment
On the day of administration, please refrain from vigorous exercise, staying up all night, excessive alcohol consumption, and the like.
Sample storage and disposal methods
The blood collected in this treatment will be used only for the patient’s own treatment. If treatment results are to be used for research purposes, separate confirmation of consent will be obtained from the patient or their proxy. Additionally, any information identifying you or your personal details will not be disclosed in any treatment outcome publications. A portion of the collected and cultured cell processed product will be stored at -80 degrees Celsius for a minimum of 10 years prior to each treatment session.
In the event of culture failure
During fat collection or during the culture of autologous adipose-derived mesenchymal stem cells, there is a very rare possibility of contamination by bacteria or fungi (contamination). If contamination is confirmed, all cultured cells will be discarded, and administration cannot proceed. If you wish to continue with the treatment, a new fat tissue collection will be required. (In this case, there is no charge for the collection.)
Approval and notification of this treatment
In accordance with the law ensuring the safety of regenerative medicine, we have undergone review by the Committee on Regenerative Medicine and obtained approval for “appropriate” treatment. Following this, we have submitted a plan for the provision of regenerative medicine to the Minister of Health, Labour and Welfare. If you wish to review the plan, please contact the inquiry desk.
Comparison with other treatment methods
Male menopause is medically referred to as Late Onset hypogonadism Syndrome (LOH Syndrome). Symptoms due to testosterone deficiency often gradually appear, and despite being symptoms of LOH Syndrome, they are sometimes misdiagnosed as “depression” or “autonomic nervous system dysfunction,” or simply dismissed as age-related changes. Consequently, appropriate treatment is often not provided.
Current treatment methods include hormone replacement therapy, traditional Chinese medicine, ED medications, psychotropic drugs or sedatives, medication for lifestyle diseases such as hypertension or dyslipidemia, and supplement therapy. However, none of these methods address the root cause of the condition.
Frequently Asked Questions
Q: Is hospitalization required?
A: It is necessary to visit the clinic twice for the collection of the patient’s own tissues, which serve as the source of the administered cells, and after cultivation is completed for administration. However, both procedures can be done as outpatient visits.
Q: How long does the treatment take?
A: The collection of the patient’s own tissues takes about 1 hour, and the treatment itself via intravenous drip takes about 1 to 2 hours, completing the procedure. Since it is by appointment only, there is no waiting time. However, it takes about 6 to 8 weeks for the cultivation of adipose-derived stem cells. Therefore, please consider the period from the initial consultation to treatment to be approximately 2 to 2.5 months.
Q: How much self-tissue is required for treatment?
A: Tissue is collected at a depth of approximately 5mm from the surface of the skin with a diameter of 3mm. Anesthesia is used during collection, so there is little pain.
Q: Is safety guaranteed?
A: Treatment via regenerative medicine requires submission of a treatment plan to the Ministry of Health, Labour and Welfare, and it cannot be provided without acceptance. Our clinic’s application has been accepted, and we offer a treatment method that has been rigorously evaluated for safety domestically.
Q: Is it eligible for medical expense deduction?
A: If it is for medical treatment purposes, it may be eligible for medical expense deduction. For details on the procedures, please contact the nearest tax office.


